IC 63 — nicknamed the Ghost Nebula — is about 550 light-years from Earth. The nebula is classified as both a reflection nebula — as it is reflecting the light of a nearby star — and as an emission nebula — as it releases hydrogen-alpha radiation. Both effects are caused by the gigantic star Gamma Cassiopeiae. The radiation of this star is also slowly causing the nebula to dissipate.
This is its energy spectrum from JS9 with its most importants energy peaks.
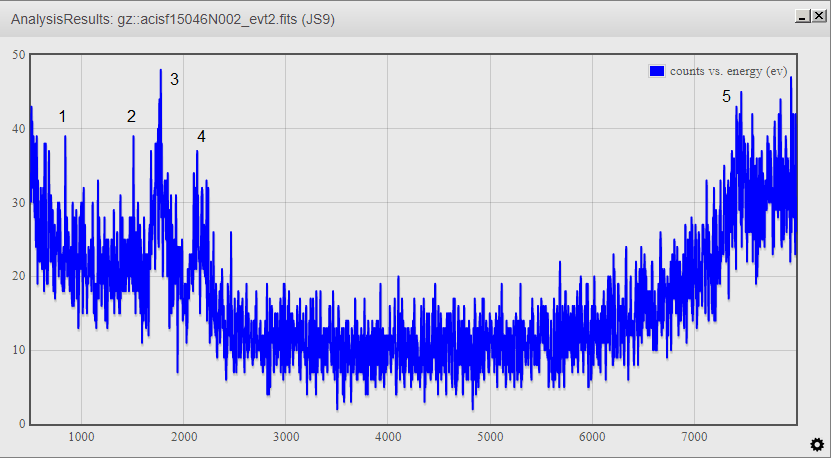
Then, I have calculated each peak's percentatge and find out which elements they contain.
I have used this formula:
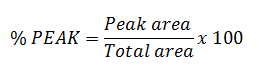 |
|
Here are my results:
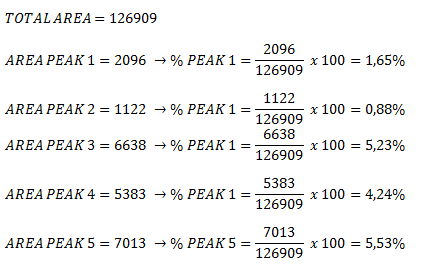 |
|
This nebula has:
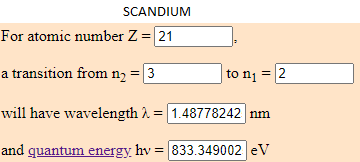
- 1,65% of Scandium (Sc) which energy is 833.3490 eV.
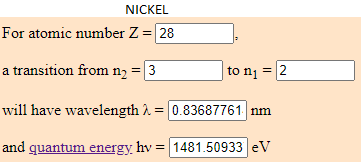
- 0,88% of Nickel (Ni) which energy is 1481.5093 eV.
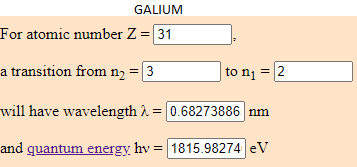
- 5,23% of Galium (Ga) which energy is 1815.9827 eV.
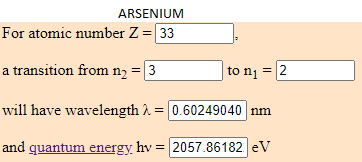
- 4,24% of Arsenic (As) which energy is 2057.8618 eV.
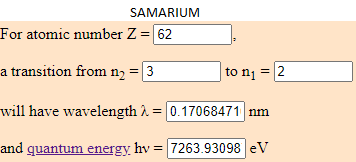
- 5,53% of Sumarium (Sm) which energy is 7263.9309 eV.
COMPARISON
I have search for scientific articles that explain which chemical elements are in IC 63. Although I have not find any that say the element it has, I have deduced that, according to Phisical and chemical structure of the IC 63 nebula by J. Jansen (1995), the nebula contains a big percentage of carbon and some oxigen and hydrogen.
In comparison with my results, they does not match with the article.
- Based on my investigation, this nebula is form of Scandium, nickel, gallium, arsenic and sumarium.
- What I have done is to find the eV of the tallest peaks of the JS9 energy spectrum and discover what element corresponds to that enery.
|
|
 |
 |
|
|
|
I have calculated the electronvolt of this elements with the transition predetermined in this web (from the cap 3 to the 2). The atoms to jump from this capes have to be in a room temperature. Depending to the temperature, the element can have different energy. What is the same, a eV number can be from different elements if they are moving arround differents capes.
It can be possible that the atoms are not moving from the capes I have work with and this might be the reason why my results does not match with the scientifics results.